pH Control
I. What is pH control in the context of Pool Water Quality?
pH is the way in which chemists measure the "acidity" of a liquid. It is measured on a scale of 1 to 14 where 1 is a very concentrated acid and 14 is extremely alkaline
pH is an abbreviation from the words “power of Hydrogen” and is a measure of the number of hydrogen molecules that have been dissolved in a litre of liquid.
In numerical terms there is a very big range of hydrogen ion concentration and so the more user friendly logarithmic scale called pH is used to measure this solution quality.
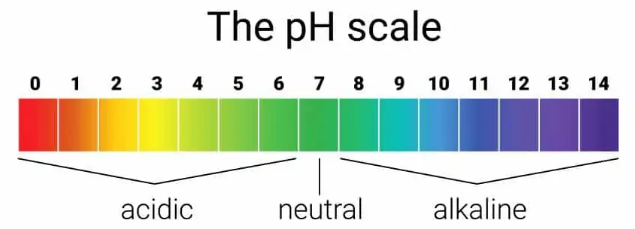
It always lies between the numbers 0 and 14 and is normally measured to one decimal place in the pool industry. At a pH value of 7.0, water is neither acidic nor alkaline but is “Neutral”. Water can become either acidic (between 0 and 6.9) or alkaline (between 7.1 and 14) when substances are added to it. For example calcium from rocks, lead from old water supply pipes, etc.
The pH value of the water in your pool will be changed by the addition of chlorine disinfectant.
II. Why does pH matter?
pH is the most important parameter to get right in your pool because it is much easier to manage the other parameters if the pH levels are between 7.0 and 7.6.
Chlorine only kills bacteria in water with a pH lower than 7.8. If the water has a pH higher than this then it does not matter how much chlorine is introduced as the bacteria will not be neutralised by the disinfectant. So it is important to keep the pH lower than 7.8 (the closer to 7.0 the better).
Equally the human body is not comfortable in water with a pH lower than 7 and so the guidelines in the UK are to keep the pH between 7.2 and 7.6 – however the lower the better.
III. pH and its effect on swimming pool chlorination
When Chlorine is added to water it forms "hypochlorous acid" and "hypochlorite". The hypochlorous acid is known in the pool industry as free chlorine and it is this “free chlorine” that actually carries out the disinfection of the pool water.
When the pH value of water is 5.5 all the chlorine added to water turns into "free chlorine" but at this level the water is far too acidic to swim in.
At a pH level of 7.0, about 80% of the added chlorine turns into "free chlorine" - but algae growth can also occur.
At a pH level of 7.2, about 50% of the added chlorine turns into “free chlorine” and this is also the pH level of our eyes, making it the most comfortable to swim in.
At a pH level of 8.0 about 20% of the added chlorine turns into “free chlorine” and so 5 times as much chlorine is required in order to disinfect the water to the same extent as for a pH of 5.5.
